Molecular hydrogen improves type 2 diabetes through inhibiting oxidative stress
https://www.spandidos-publications.com/10.3892/etm.2020.8708?fbclid=IwAR0WhfjO_KOGpJQIaDcE6ZtqNGTR5Uc9XZ1ik65jzRh1mW96RG1Gzb6TBZk
Molecular hydrogen improves type 2 diabetes through inhibiting oxidative stress
- Authors:
- Published online on: April 30, 2020 https://doi.org/10.3892/etm.2020.8708
- Pages: 359-366
Copyright: © Ming et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY_NC 4.0].
Abstract
Introduction
Diabetes mellitus (DM), characterized by chronic hyperglycemia, is a multi-factorial metabolic disorder that is also gaining recognition as a chronic inflammatory disease (1). Physiological reactive oxygen species (ROS) is essential for the maintenance and regulation of a number of important physiological processes, including cell proliferation, apoptosis and Ca2+ signaling (2). However, excessive ROS production can induce damage to proteins, DNA and lipids caused by oxidative stress (3). Hyperglycemia increases the intracellular NADH/NAD+ ratio, increasing the risk of electron leakage from NADH or FADH2 of the electron transport chain, in turn resulting in increased ROS production (4). Additionally, hyperglycemic states can also decrease the expression and activity of enzymes that eliminate ROS, further aggravating oxidative stress (5). Previous studies have demonstrated that excessive ROS accumulation in the mitochondria induced by hyperglycemia can cause damage to the mitochondrial inner membrane. Firstly, ROS can increase inner membrane fluidity and permeability, adversely altering mitochondrial osmotic pressure, causing the organelle to swell; secondly, ROS can destroy the mitochondrial membrane potential and in turn reduce ATP synthesis (6,7). Downstream, this can promote the efflux of apoptotic factors from the mitochondrial matrix and activate apoptotic pathways in the cytosol, resulting in islet cell and renal podocyte apoptosis (8,9).
In recent years, it has become evident that Hydrogen (H2) is an effective treatment for various disease models such as myocardial ischemia-reperfusion injury, COPD, and lipid metabolism disorders (10-12). Although previous studies in rats have revealed that hydrogen production exhibited beneficial therapeutic effects on early islet cells, the mechanism underlying this protection remains poorly understood (13,14). Therefore, in the present study, using rat models with type 2 DM (T2DM) induced by high-fat feeding combined with low-dose streptozotocin (STZ) injection, the potential therapeutic effects of H2 in T2DM were evaluated. Particular emphasis was placed on glucose metabolism, insulin resistance, lipid metabolism, oxidative stress and histological morphology along with the associated molecular mechanisms. The present study aimed to provide further understanding for the clinical application of H2 in the treatment of T2DM.
Materials and methods
Preparation and grouping of diabetic rat models
The present study was approved by the animal ethics committee of Weifang Peoples Hospital (approval no. WFPH2016011K; Weifang, China). A total of 50 Specific-pathogen-free Sprague Dawley rats (2 months old; male:female=1:1), weighing 200±20 g, were purchased from Weifang People's Hospital (license no. WFPH2016011K). The animals were maintained at 22-25˚C and a relative humidity of 40-70% with a 12-h light/dark cycle and provided ad libitum access to food and drinking water. Following normal feed for 3 days, rats were randomized into the following two groups: i) High fat (n=40); and ii) normal (n=10; male:female, 1:1). The high-fat feed composed of 59% common feed, 10% lard, 10% yolk powder, 20% sucrose and 1% cholesterol, which was provided by Weifang People's Hospital. After 4 weeks of this high-fat diet, animals in the T2DM model group received a tail vein injection of 30 mg/kg STZ (Sigma-Aldrich; Merck KGaA), following which fasting blood glucose concentrations of the rats were measured 1 week later. Rats with fasting blood glucose concentrations <16.7 mmol/l received a second dose of STZ (30 mg/kg) iimmediately after glucose measurements through tail veins prior to another round of fasting blood glucose measurements 1 week later. Rats with fasting blood glucose concentrations >16.7 mmol/l were considered to be diabetic (15). Diabetic model rats were subsequently randomized into three groups (n=10 in each group; male:female, 1:1): i) H2; ii) positive control (300 mg/kg metformin via intragastric injection; Sino-American Shanghai Squibb Pharmaceutical Co., Ltd.); and iii) model (equivalent volume of physiological saline). During the H2 administration period, diabetic rats in the H2 group were provided with 500 µl saturated hydrogen saline by intragastric injection. In addition, a high-fat diet control group (n=10; male:female, 1:1) was set, where the animals were fed on a high-fat diet for 4 weeks followed by the intragastric delivery of an equivalent volume of physiological saline parallel to H2 administration; during the H2 administration period, rats in the high-fat diet control group received an equivalent volume of physiological saline. Rats in the normal group (n=10) were fed with common feed for 4 weeks, following which they were injected with an equal volume of physiological saline via the tail vein; during the H2 administration period, rats in the normal group received an equivalent volume of physiological saline. All rats were treated with either H2, metformin or physiological saline daily for 80 consecutive days. The body weights of all animals were recorded after every 2 weeks. Saturated hydrogen saline was prepared in the Center of Modern Analysis and Detection of Xi'an Jiaotong University. Molecular H2 was dissolved in normal saline at high pressure (13.5 Mpa) to form a saturated solution, which was subsequently stored in aluminum packaging to maintain the H2 concentration at >0.6 mmol/l.
Preparation of samples
On day 0 of treatment, following 12 h fasting, blood samples (0.5-1 ml) were obtained from the tail veins of rats from each group under anesthesia with 4% diethyl ether. Following centrifugation at 4˚C and 1,800 x g for 15 min, the supernatants were collected for the measurement of biochemical indicators. On day 80, after 8 h fasting, the rats were euthanized following an intravenous injection of 100 mg/kg sodium pentobarbital, following which 1 ml blood samples were taken from the hepatic portal veins of rats in each group. Following centrifugation at 4˚C and 1,800 x g for 15 min, the supernatants were collected for the measurement of biochemical indicators. Subsequent to euthanasia, the kidneys and pancreas tissues were harvested from the rats in each group, rinsed with saline and dried using filter paper. The tissues were then fixed with 10% formaldehyde at 4˚C for 24 h. After gradient ethanol elution, xylene transparentizing and paraffin embedding, tissues were cut into 2-5 µm sections. The sections were mounted on glass slides and baked for 45 min at 80˚C and treated with xylene I and xylene II (Tiangen Biotech Co. Ltd.) for 20 min. Samples were then incubated at room temperature with 95, 85 and 75% alcohol (3 min for each concentration). Sections were stained with haematoxylin for 60 sec and eosin for 30 sec (Sigma-Aldrich; Merck KGaA) at room temperature. Sections were sealed using neutral gum and observed under inverted microscope (Model, IX70; Olympus Corporation).
Measurement of biochemical indicators
To evaluate the efficacy of H2 for the treatment of T2DM, fasting blood glucose, hepatic glycogen, fasting serum insulin, insulin sensitivity index, insulin resistance index, serum superoxide dismutase (SOD) and serum malondialdehyde (MDA) were measured in samples collected from rats in each group. Serum glucose was assayed using glucose oxidase-peroxidase method, whereas hepatic glycogen was measured using the anthraquinone method. Serum insulin was assayed by double antibody sandwich ELISA, serum SOD was measured using the WST-1 method, which is based on the cleavage of the tetrazolium salt WST-1 to formazan by SOD. Serum MDA was measured using the 2-thiobarbituric acid (TBA) method, which is based on the colored substances produced by interactions between MDA and TBA. All assays were performed according to the protocols of the respective manufacturers. Glucose assay kits (cat. no. F006-1-1), SOD kits (cat. no. A001-3-2), MDA assay kits (cat. no. A003-1-2), hepatic/muscle glycogen kits (cat. no. A043-1-1) were purchased from Nanjing Jiancheng Bioengineering Institute, whilst the rat insulin ELISA kits (cat. no. 10-1250-01) were purchased from Mercodia AB.
Measurement of blood lipids
An automatic biochemical analyzer (AU680; Beckman Coulter, Inc.) was used to measure triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c) and low-density lipoprotein cholesterol (LDL-c) levels in the blood samples collected from each rat. Each condition was examined in triplicate in parallel and were subsequently averaged. TC, TG, HDL-c and LDL-c kits were purchased from Roche Diagnostics. Test procedures for each item were performed in strict accordance with the manufacturer's protocols.
Calculation of insulin resistance
Insulin resistance was calculated using values obtained from fasting blood glucose and fasting insulin measurements in accordance with the following formula: Insulin resistance index (IRI)=(fasting blood glucose x fasting insulin)/22.5(16).
Western blotting
Pancreatic tissues (1 mg) were lysed using RIPA buffer (Beijing Dingguo Changsheng Biotechnology Co., Ltd.) supplemented with proteinase inhibitor for 30 min on ice and subsequently centrifuged at 12,000 x g at 4˚C for 15 min. Protein concentration was quantified using bicinchoninic acid assay protein quantitative kit (Beyotime Institute of Biotechnology). Protein (50 µg) was separated by 10% SDS-PAGE prior to transferal onto PVDF membranes. The membranes were then blocked using 5% skim milk at room temperature for 1 h. Primary antibodies (all obtained from Abcam) against toll-like receptor 4 (TLR4; 1:400; cat. no. ab13556), myeloid differentiation primary response 88 (MyD88; 1:400; cat. no. ab2064), phosphorylated (p)-p65 (1:500; cat. no. ab97726), p65 (1:500; cat. no. ab32536), p-IκB (1:500; cat. no. ab133462), IκB (1:1,000; cat. no. ab12134) and β-actin (1:2,000; cat. no. ab8226) were subsequently used to incubate the membranes overnight at 4˚C. The membranes were then incubated with horseradish peroxidase-conjugated goat anti-rabbit antibodies (cat. no. BA1055; 1:2,000; Boster Biological Technology) at 37˚C for 1 h. ECL (Beijing Dingguo Changsheng Biotechnology Co., Ltd.) reagent was used to visualize the protein bands. Quantity One Software (version 4.6.9; Bio-Rad Laboratories, Inc.) was used to perform quantitative densitometric analysis (17) where β-actin was used as loading control.
Statistical analysis
Experimental data in each group were presented as mean ± standard deviation; sample means among groups were compared using one-way ANOVA followed by Tukey's test for all data using SPSS 18.0 software (SPSS Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
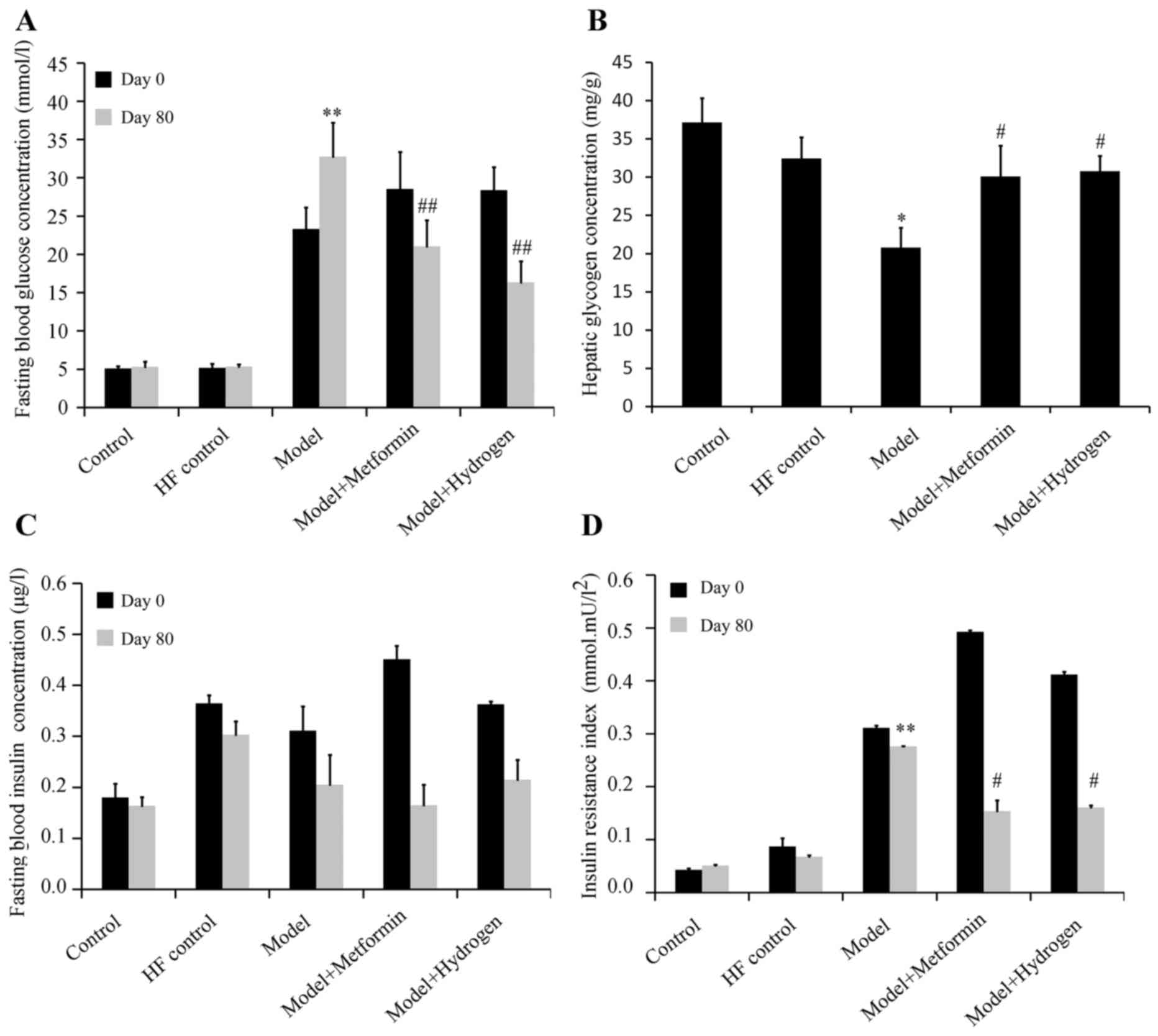
H2 can effectively restore blood glucose levels following T2DM induction
The effect of H2 on fasting blood glucose levels in rats following the induction of T2DM is shown in Fig. 1A. The mean fasting blood glucose levels were significantly increased in the model group on both days 0 and 80 when compared with the HF control. On day 80, fasting blood glucose levels in the H2 group were significantly decreased compared with the model group. On the same day, hepatic glycogen content in the H2 group was significantly increased compared with that in the model group (Fig. 1B). The effect of H2 on fasting serum insulin levels in rats following the induction of T2DM is shown in Fig. 1C. Compared with the model group, no significant differences were observed in the fasting serum insulin levels on days 0 or 80 in the H2 group. The effect of H2 on insulin sensitivity in rats following the induction of T2DM is presented in Fig. 1D. On day 80, compared with the normal group, IRI was significantly increased in the model group, whilst the IRI was significantly decreased in H2 group compared with the model group.
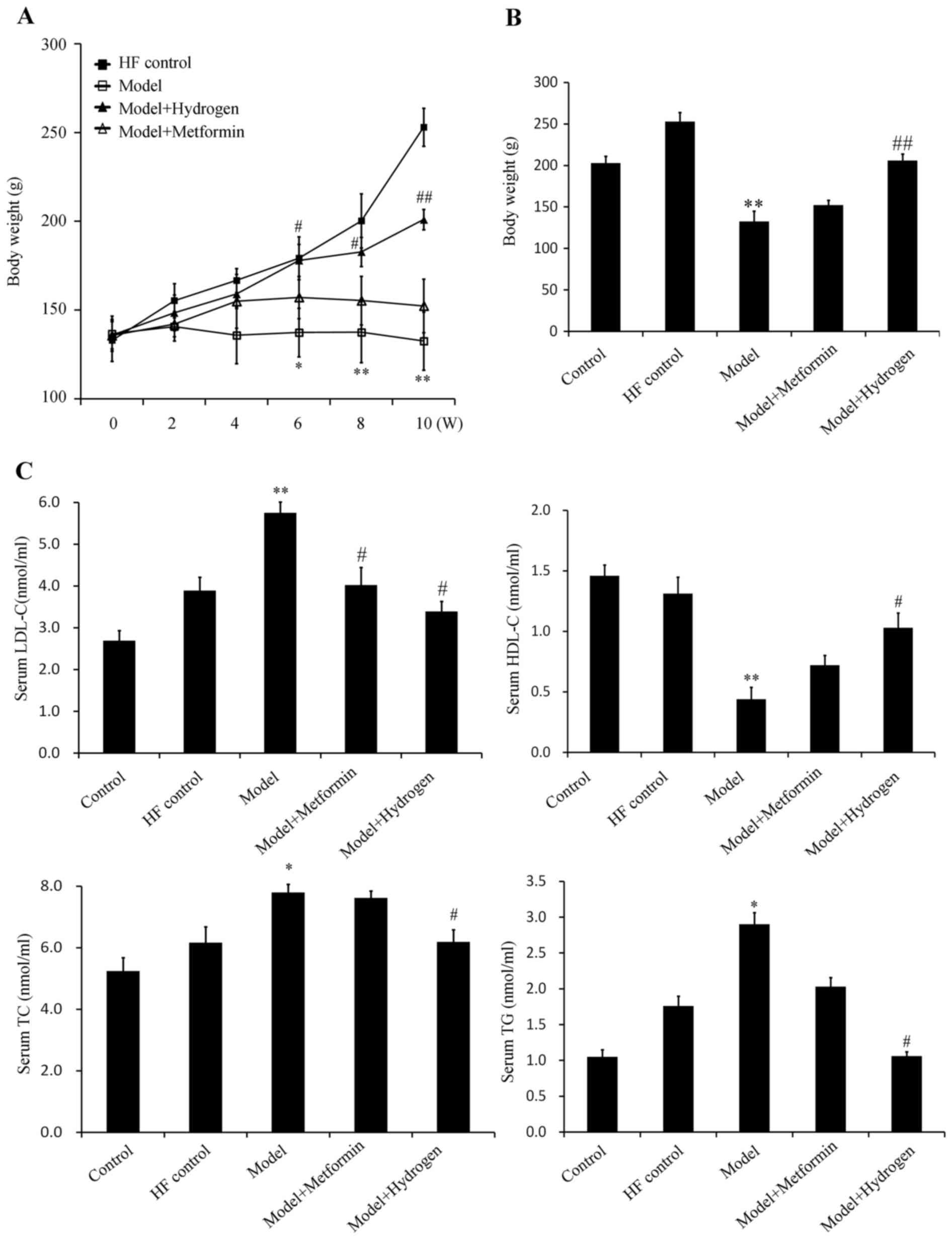
H2 can effectively restore blood lipid levels following T2DM
The average weights of the rats in the model group were significantly decreased compared with the HF control group from week 6 onwards, whilst the average weights of the rats in the H2 group were significantly increased compared with those in the model group (Fig. 2A and B). The serum levels of TG, TC and LDL-c in the model group were significantly increased compared with those in the HF control group, whereas the serum levels of HDL-c in the model group were significantly decreased compared with those in the HF control group (Fig. 2C). In the H2 group, the TG, TC and LDL-c levels were significantly decreased, whereas the levels of HDL-c were significantly increased compared with those in the model group (Fig. 2C). The corresponding levels of TG, TC and HDL-c in the positive control metformin group exhibited no protective effects, suggesting that H2 was more effective in restoring the levels of blood lipids following T2DM induction.
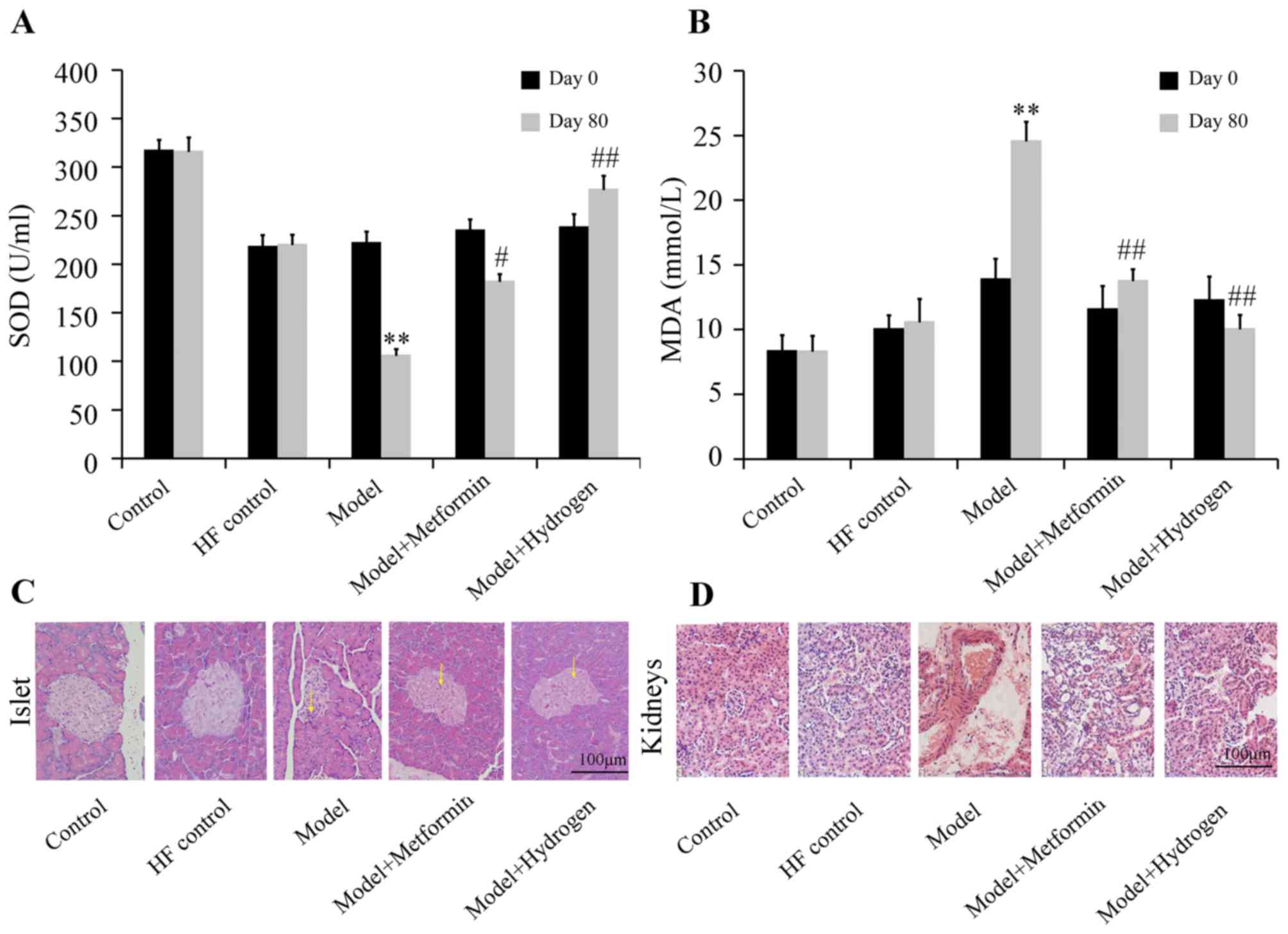
H2 can alleviate oxidative stress and pathological morphology of T2DM
The effect of H2 on SOD levels of rats with T2DM was shown in Fig. 3A. Compared with the HF control group, SOD levels in the model group were significantly decreased on day 80, whereas serum SOD content in the H2 group was significantly increased compared with the model group. Additionally, serum MDA concentrations were significantly decreased in the H2 group on day 80 compared with those from the model group, which was in turn significantly increased compared with the HF control group (Fig. 3B).
H&E staining results of pancreatic islets collected on day 80 are shown in Fig. 3C. In the normal group, the islets were morphologically complete with clearly visible boundaries between islets and exocrine glands, where the number of cells in the islets was large and the cells were densely and uniformly arranged. In the model group, the islets were irregular in morphology, where the boundaries between the islets and exocrine glands became ambiguous and the quantity of cytoplasm in islet cells was decreased; some islet cells also exhibited vacuolar degeneration in the cytoplasm. In the H2 group, the islets were complete in morphology with clear boundaries between islets and exocrine glands, where the quantity of cytoplasm in islet cells was markedly increased with decreased vacuolar degeneration compared with those in the model group. H&E staining results of the glomeruli are shown in Fig. 3D. The glomeruli of the rats in the normal group were complete in morphology with clear contours and regular arrangement, where no abnormalities were observed in the tubules. In the model group, capsular spaces were narrowed with tubular structures exhibiting disorder. In the H2 group, the glomeruli were morphologically complete with regularly arranged structures, where the tubules clearly visible, comparable with those observed in the control group.
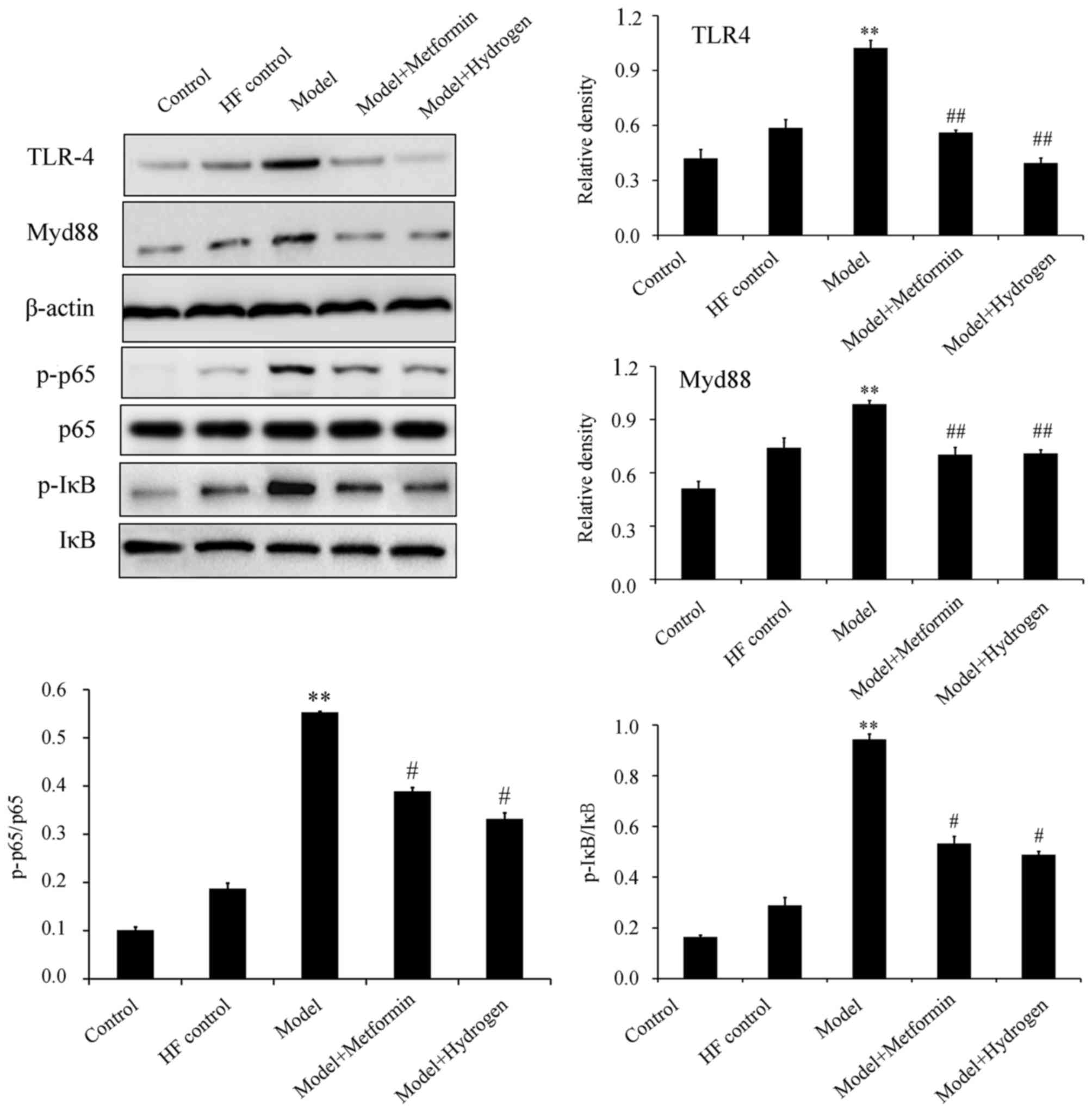
H2 can inhibit TLR4/MyD88/NF-κB signaling
The TLR4/NF-κB signaling pathway has been previously demonstrated to serve an important role in STZ-induced diabetic rats fed on a high-fat diet (18). Western blot analysis revealed that H2 treatment significantly inhibited the expression levels of TLR4 and MyD88, in addition to significantly decreasing p65 and IκB phosphorylation in pancreatic tissues isolated on day 80 compared with the model group (Fig. 4).
Discussion
In the present study, to mimic the progression of T2DM in humans, rat models of T2DM induced by a high-fat diet followed by low-dose STZ injection were generated. Compared with the rats with spontaneous diabetes, model animals such as those used in the present study are readily available, are more cost effective and therefore have been used for evaluating drug efficacy for T2DM (19,20). In the present study, compared with the HF control group, the model group exhibited significantly increased fasting blood glucose levels and insulin resistance, though no significant difference was observed in fasting serum insulin levels. In addition, compared with those in the HF control group, the levels of TG, TC and LDL-c were significantly increased, whilst the levels of HDL-c were significantly decreased in the model group. Serum SOD and MDA concentrations were significantly decreased and increased on day 80 in the model group compared with those in the HF control group, respectively. Therefore, these results suggest that a high-fat + high-sugar diet followed by low-dose STZ injection can induce the symptoms of hyperglycemia, hyperlipemia and oxidative stress in rats, making it a suitable animal model for investigating drug efficacy for anti-diabetic treatments.
In the present study, changes in glucose metabolism, insulin resistance, lipid metabolism and oxidative stress in diabetic rats were observed following intragastric H2 administration. Typically, blood glucose is used for oxidative phosphorylation in most tissues, converted into hepatic or muscle glycogen for storage, converted to other sugars and derivatives or non-sugar substances for use in other pathways and excreted by urine if blood glucose concentration becomes too high (21). In the present study, glycogen levels in the blood isolated from the hepatic portal vein was higher in the H2 group, suggesting that H2 can promote the synthesis of hepatic glycogen whilst improving the utilization of glucose in the liver and lower fasting blood sugar concentration. Insulin resistance is a core characteristic in T2DM pathophysiology that is closely associated with lipid metabolism disturbance, damage to islet β-cells and finally islet failure (22). Therefore, it is of upmost importance to delay T2DM progression by improving insulin resistance. Data from the present study suggested that H2 treatment did not affect insulin concentration whilst improving insulin sensitivity in diabetic rats, alleviating the symptoms of insulin resistance.
Previous studies have revealed aberrant lipid metabolism in patients with T2DM, where high blood glucose levels, insulin resistance and hyperinsulinemia all contribute to the dysfunction of lipoprotein metabolism in the body (23,24). The present study revealed that the levels of TG, TC and LDL-c in the model group were significantly increased compared with those in the HF control group, whilst the levels of HDL-c in the model group were significantly decreased compared with those in the HF control group. H2, but not the positive control metformin, exerted protective effects on blood lipids during T2DM induction, suggesting that H2 can reverse the dysfunction in blood lipid metabolism during T2DM pathogenesis.
Oxidative stress is an important cause of diabetes and its associated complications (25). Metabolism under hyperglycemic conditions leads to the production of excessive quantities of superoxides and the inactivation of antioxidants in the body (26). Therefore, ROS oxidative stress serves an important role in the pathogenesis of diabetes (27). The effects of H2 on oxidative stress have been previous reported (28,29). The present study demonstrated that H2 can increase SOD activity whilst decreasing serum MDA content.
TLR4 and one of its endogenous ligands, MyD88, are frequently upregulated in glomeruli of type 1 (STZ-induced) and type 2 (A-ZIP/F-1 lipoatrophic) diabetic mice (30). Activation of TLR4/MyD88 signaling was also previously revealed in an animal model of diabetic glomerular injury accompanied with hyperlipidemia (31). NF-κB is a key transcription factor that initiates immune responses and activates the expression of inflammatory cytokines during oxidative stress downstream of TLR4/MyD8 signaling (32). At resting state, NF-κB exists in the cytoplasm as an inactive NF-κB/IκBα complex. Following activation, IκBα is phosphorylated and subsequently degraded. Following the dissociation of NF-κB and IκBα, NF-κB translocate into the nucleus and activate the transcription of genes associated with inflammation, including nitric oxide, tumor necrosis factor-α, interleukin (IL)-1β and IL-6(33). The present study suggested that H2 can effectively suppress the activation of TLR4/MyD88/NF-κB during T2DM.
In conclusion, H2 is effective for treating T2DM by alleviating hyperglycemia, hyperlipemia and antioxidant capacity by suppressing TLR4/MyD88/NF-κB signaling. However, further in vivo and in vitro experiments are required to verify the effects of H2 on sugar and lipid metabolism and NF-κB/IκB signaling.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Weifang Science and Technology Development Plan (grant no. 201202153).
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Authors' contributions
YM and QHM performed the experiments. XH performed statistical analysis. HYL deigned the current study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the animal ethics committee of Weifang Peoples Hospital (approval no. WFPH2016011K; Weifang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
Moradi F, Maleki V, Saleh-Ghadimi S, Kooshki F and Pourghassem Gargari B: Potential roles of chromium on inflammatory biomarkers in diabetes: A Systematic. Clin Exp Pharmacol Physiol. 46:975–983. 2019.PubMed/NCBI View Article : Google Scholar | |
Bevilacqua E, Gomes SZ, Lorenzon AR, Hoshida MS and Amarante-Paffaro AM: NADPH oxidase as an important source of reactive oxygen species at the mouse maternal-fetal interface: Putative biological roles. Reprod Biomed Online. 25:31–43. 2012.PubMed/NCBI View Article : Google Scholar | |
Burtenshaw D, Kitching M, Redmond EM, Megson IL and Cahill PA: Reactive Oxygen Species (ROS), intimal thickening, and subclinical atherosclerotic disease. Front Cardiovasc Med. 6(89)2019.PubMed/NCBI View Article : Google Scholar | |
Schönfeld P: Can all major Ros forming sites of the respiratory chain be activated by high FADH2/NADH Ratios? Bioessays. 41(e1800225)2019.PubMed/NCBI View Article : Google Scholar | |
Lee JY, Lee YJ, Jeon HY, Han ET, Park WS, Hong SH, Kim YM and Ha KS: The vicious cycle between transglutaminase 2 and reactive oxygen species in hyperglycemic memory-induced endothelial dysfunction. FASEB J. 33:12655–12667. 2019.PubMed/NCBI View Article : Google Scholar | |
Bravard A, Bonnard C, Durand A, Chauvin MA, Favier R, Vidal H and Rieusset J: Inhibition of xanthine oxidase reduces hyperglycemia-induced oxidative stress and improves mitochondrial alterations in skeletal muscle of diabetic mice. Am J Physiol Endocrinol Metab. 300:E581–E591. 2011.PubMed/NCBI View Article : Google Scholar | |
Cadenas S: Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim Biophys Acta Bioenerg. 1859:940–950. 2018.PubMed/NCBI View Article : Google Scholar | |
Sifuentes-Franco S, Padilla-Tejeda DE, Carrillo-Ibarra S and Miranda-Díaz AG: Oxidative stress, apoptosis, and mitochondrial function in diabetic nephropathy. Int J Endocrinol. 2018(1875870)2018.PubMed/NCBI View Article : Google Scholar | |
Ni Z, Tao L, Xiaohui X, Zelin Z, Jiangang L, Zhao S, Weikang H, Hongchao X, Qiujing W and Xin L: Polydatin impairs mitochondria fitness and ameliorates podocyte injury by suppressing Drp1 expression. J Cell Physiol. 232:2776–2787. 2017.PubMed/NCBI View Article : Google Scholar | |
Hayashida K, Sano M, Ohsawa I, Shinmura K, Tamaki K, Kimura K, Endo J, Katayama T, Kawamura A, Kohsaka S, et al: Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun. 373:30–35. 2008.PubMed/NCBI View Article : Google Scholar | |
Lu W, Li D, Hu J, Mei H, Shu J, Long Z, Yuan L, Li D, Guan R, Li Y, et al: Hydrogen gas inhalation protects against cigarette smoke-induced COPD development in mice. J Thorac Dis. 10:3232–3243. 2018.PubMed/NCBI View Article : Google Scholar | |
Qiu X, Ye Q, Sun M, Wang L, Tan Y and Wu G: Saturated hydrogen improves lipid metabolism disorders and dysbacteriosis induced by a high-fat diet. Exp Biol Med. 245:512–521. 2020.PubMed/NCBI View Article : Google Scholar | |
Taniguchi S, Kang L, Kimura T and Niki I: Hydrogen sulphide protects mouse pancreatic β-cells from cell death induced by oxidative stress, but not by endoplasmic reticulum stress. Br J Pharmacol. 162:1171–1178. 2011.PubMed/NCBI View Article : Google Scholar | |
Ali MY, Whiteman M, Low CM and Moore PK: Hydrogen sulphide reduces insulin secretion from HIT-T15 cells by a KATP channel-dependent pathway. J Endocrinol. 195:105–112. 2007.PubMed/NCBI View Article : Google Scholar | |
Lin S, Yang J, Wu G, Liu M, Lv Q, Yang Q and Hu J: Inhibitory effects of taurine on STZ-induced apoptosis of pancreatic islet cells. Adv Exp Med Biol. 775:287–297. 2013.PubMed/NCBI View Article : Google Scholar | |
Belfiore F, Iannello S, Camuto M, Fagone S and Cavaleri A: Insulin sensitivity of blood glucose versus insulin sensitivity of blood free fatty acids in normal, obese, and obese-diabetic subjects. Metabolism. 50:573–582. 2001.PubMed/NCBI View Article : Google Scholar | |
Qin L, Qiu K, Hu C, Wang L, Wu G and Tan Y: Respiratory syncytial virus promoted the differentiation of Th17 cells in airway microenvironment through activation of Notch-1/Delta3. J Med Microbiol. 68:649–656. 2019.PubMed/NCBI View Article : Google Scholar | |
Ma ZJ, Zhang XN, Li L, Yang W, Wang SS, Guo X, Sun P and Chen LM: Tripterygium glycosides tablet ameliorates renal tubulointerstitial fibrosis via the toll-like receptor 4/nuclear factor kappa B signaling pathway in high-fat diet fed and streptozotocin-induced diabetic rats. J Diabetes Res. 2015(390428)2015.PubMed/NCBI View Article : Google Scholar | |
Zhang F, Yuan W, Wei Y, Zhang D, Duan Y, Li B, Wang X, Xi L, Zhou Y and Wu X: The alterations of bile acids in rats with high-fat diet/streptozotocin-induced type 2 diabetes and their negative effects on glucose metabolism. Life Sci. 229:80–92. 2019.PubMed/NCBI View Article : Google Scholar | |
Zhang Y, Zhou G, Peng Y, Wang M and Li X: Anti-hyperglycemic and anti-hyperlipidemic effects of a special fraction of Luohanguo extract on obese T2DM rats. J Ethnopharmacol. 247(112273)2020.PubMed/NCBI View Article : Google Scholar | |
Xing HY, Cai YQ, Wang XF, Wang LL, Li P, Wang GY and Chen JH: The Cytoprotective effect of hyperoside against oxidative stress is mediated by the Nrf2-ARE signaling pathway through GSK-3β inactivation. PLoS One. 10(e0145183)2015.PubMed/NCBI View Article : Google Scholar | |
Corsa CAS, Pearson GL, Renberg A, Askar MM, Vozheiko T, MacDougald OA and Soleimanpour SA: The E3 ubiquitin ligase parkin is dispensable for metabolic homeostasis in murine pancreatic β cells and adipocytes. J Biol Chem. 294:7296–7307. 2019.PubMed/NCBI View Article : Google Scholar | |
Aslam M, Aggarwal S, Sharma KK, Galav V and Madhu SV: Postprandial hypertriglyceridemia predicts development of insulin resistance glucose intolerance and type 2 diabetes. PLoS One. 11(e0145730)2016.PubMed/NCBI View Article : Google Scholar | |
Qiu J, Liu Y, Yue Y, Qin Y and Li Z: Dietary tartary buckwheat intake attenuates insulin resistance and improves lipid profiles in patients with type 2 diabetes: A randomized controlled trial. Nutr Res. 36:1392–1401. 2016.PubMed/NCBI View Article : Google Scholar | |
Álvarez-Almazán S, Filisola-Villaseñor JG, Alemán-González-Duhart D, Tamay-Cach F and Mendieta-Wejebe JE: Current molecular aspects in the development and treatment of diabetes. J Physiol Biochem. 76:13–35. 2020.PubMed/NCBI View Article : Google Scholar | |
Kikuchi C, Kajikuri J, Hori E, Nagami C, Matsunaga T, Kimura K and Itoh T: Aortic superoxide production at the early hyperglycemic stage in a rat type 2 diabetes model and the effects of pravastatin. Biol Pharm Bull. 37:996–1002. 2014.PubMed/NCBI View Article : Google Scholar | |
Abbasihormozi SH, Babapour V, Kouhkan A, Niasari Naslji A, Afraz K, Zolfaghary Z and Shahverdi AH: Stress hormone and oxidative stress biomarkers link obesity and diabetes with reduced fertility potential. Cell J. 21:307–313. 2019.PubMed/NCBI View Article : Google Scholar | |
LeBaron TW, Kura B, Kalocayova B, Tribulova N and Slezak J: A new approach for the prevention and treatment of cardiovascular disorders. Molecular hydrogen significantly reduces the effects of oxidative stress. Molecules. 24(pii: E2076)2019.PubMed/NCBI View Article : Google Scholar | |
Yuan J, Wang D, Liu Y, Chen X, Zhang H, Shen F, Liu X and Fu J: Hydrogen-rich water attenuates oxidative stress in rats with traumatic brain injury via Nrf2 pathway. J Surg Res. 228:238–246. 2018.PubMed/NCBI View Article : Google Scholar | |
Kuwabara T, Mori K, Mukoyama M, Kasahara M, Yokoi H and Nakao K: Macrophage-mediated glucolipotoxicity via myeloid-related protein 8/toll-like receptor 4 signaling in diabetic nephropathy. Clin Exp Nephrol. 18:584–592. 2014.PubMed/NCBI View Article : Google Scholar | |
Kuwabara T, Mori K, Mukoyama M, Kasahara M, Yokoi H, Saito Y, Ogawa Y, Imamaki H, Kawanishi T, Ishii A, et al: Exacerbation of diabetic nephropathy by hyperlipidaemia is mediated by Toll-like receptor 4 in mice. Diabetologia. 55:2256–2266. 2012.PubMed/NCBI View Article : Google Scholar | |
Zhang JS, Tan YR Xiang Y, Luo ZQ and Qin XQ: Regulatory peptides modulate adhesion of polymorphonuclear leukocytes to bronchial epithelial cells through regulation of interleukins, ICAM-1 and NF-kappaB/IkappaB. Acta Biochim Biophys Sin (Shanghai). 38:119–128. 2006.PubMed/NCBI View Article : Google Scholar | |
Tan Y, Liu H, Yang H, Wang L and Qin X: An inactivated Pseudomonas aeruginosa medicament inhibits airway allergic inflammation and improves epithelial functions. J Physiol Sci. 63:63–69. 2013.PubMed/NCBI View Article : Google Scholar |
Related Articles
- Hyperbaric oxygen treatment improves pancreatic β‑cell function and hepatic gluconeogenesis in STZ‑induced type‑2 diabetes mellitus model mice
- Effects of basal insulin application on serum visfatin and adiponectin levels in type 2 diabetes
- Analysis of the association between adiponectin, adiponectin receptor 1 and diabetic cardiomyopathy
- Protective effect of carvacrol on liver injury in type 2 diabetic db/db mice
- Effect of oxymatrine on liver gluconeogenesis is associated with the regulation of PEPCK and G6Pase expression and AKT phosphorylation